You are here
What is CML?
Normally each of us inherits 23 pairs of ‘homologous’ chromosomes (twin pairs with the same basic structure) one set from our father and one set from our mother, making 46 chromosomes in total. Each pair is numbered from 1 to 22 according to size. Number 23 is the pair that defines gender, containing either an X and a Y chromosome in men, or two X chromosomes in women.
All 23 pairs are found in the nucleus or ‘engine’ of all blood cells apart from red cells.
Changes to the basic structure of chromosomes can sometimes occur, possibly through exposure to ionizing radiation, environmental toxins or for other unknown reasons.
Chromosomes are not visible in the nucleus unless the cell is in its dividing phase. They are actually long strands of DNA which, during the process of cell division become tightly packed; only then are they visible under a microscope. Most of what we know about chromosomes was learned by researchers observing cells during the dividing cycle.
What is the Philadelphia Chromosome?
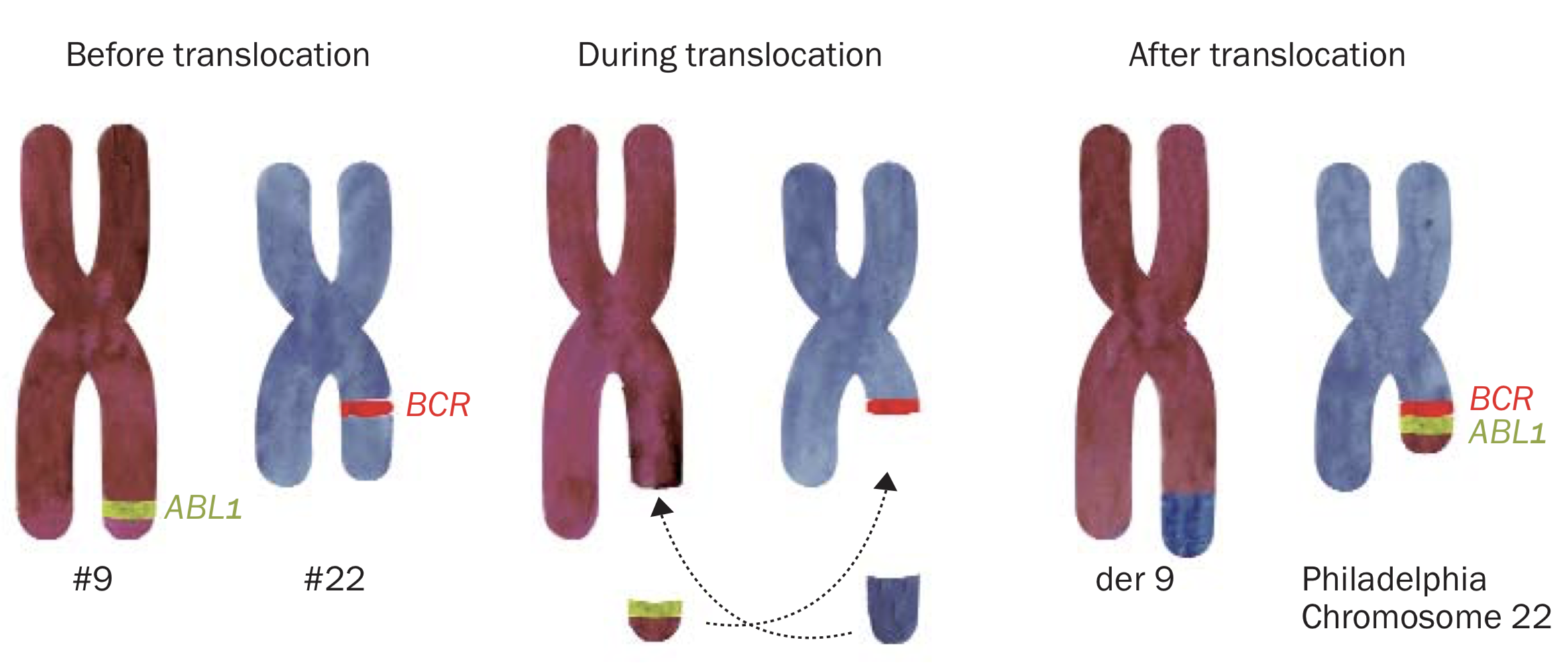
The Philadelphia chromosome is an acquired abnormality and is the definitive marker for CML. It is formed when chromosomes 9 and 22 swap one part each with the other. It is not yet understood why this happens. CML is not an inherited condition and as such cannot be passed on to children.
Known as a reciprocal translocation, a piece containing the ABL1 gene from the bottom part of chromosome 9 breaks off and attaches to a region on chromosome 22 where the BCR gene is located, thus forming a new chromosome 22 containing the abnormal fusion gene BCR-ABL1
The new shortened chromosome 22 is called the Philadelphia chromosome. You might also see it written as t(9; 22). The BCR-ABL1. fusion gene makes a protein called a tyrosine kinase. These kinds of proteins are located on or near the surface of cells and send signals that speed up cell division.
Ph+CML is a rare disease with an incidence of 1 to 1.5 new cases per 100.000 people each year. It is very rare in young people under 19 and ultra-rare in young children.
It has been shown that the BCR-ABL1 fusion gene is the single definitive marker for Ph+ CML and remains the key abnormality throughout the chronic phase of the disease.
The BCR-ABL1 fusion gene translates as a protein tyrosine kinase, referred to as Bcr-Abl1. The activity of tyrosine kinases is typically controlled by other molecules, but the mutant tyrosine kinase produced by the BCR-ABL1 gene means the signal for cell division is always ‘switched on’. Its continuous activity sets up a cascade of events that allows for unregulated cell division (cancer). Over time the Ph+ cells overpopulate the marrow, crowding out normally functioning cells.
Since the introduction of targeted therapy with tyrosine kinase inhibitors (TKIs), it is increasingly evident that, at least in chronic phase, when cells containing the BCR-ABL1 gene are reduced to a molecular level, (0.1% or lower) the risk of disease progression is significantly reduced.