Hi Nicola
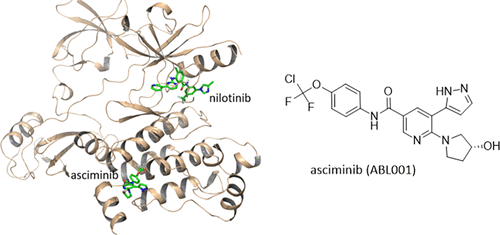
I posted here on forum previously about Asciminib and how it had been presented at the ASH conference in US in Nov/Dec 2020 and Scuba posted one of the academic papers from Jorge Cortes and others.Basically everyone thought that it was a new and novel tki that would take the place of those who had failed bosutinib /ponatinib.
I think that the path for approval would be to FDA for consideration and then the UK via NICE would consider it;because of Brexit I think that the European Medicines Agency would now be redundant-except for Northern Ireland.
So I suggest pending approval by NICE which could take some time as it is manufactured by Novartis who have a base in UK why dont you suggest to your clinicians to approach the manufacturer to see if it is available under their patient access scheme.The problem is that until it is approved by FDA/NICE it is still classed as an experimental drug.Patient access schemes are normally targeted to developing countries that cannot afford expensive tkis .You might also check if there any upcoming trials with Asciminib in UK.
Regards
John